Acthar Gel Nephrotic Syndrome
Acthar gel nephrotic syndrome. Has anyone had experience with Acthar Gel. Sometimes the cause is unknown. Most of what I see appears to say its super expensive but I have an opportunity to participate in a study so that wouldnt be an issue.
Acthar Gel may help appropriate patients with proteinuria in nephrotic syndrome due to FSGS Image is not an actual patient. My bigger concern is side effects. Conclusions Acthar gel may meet an important treatment need in patients with treatment-resistant NS in response to first-line therapies patients unable to tolerate first-line therapies and in.
Acthar Gel adrenocorticotropic hormone repository corticotropin injection Mallinckrodt ARD Inc Hazelwood MO FDA-approved in the US to induce diuresis or remission of proteinuria in NS without uremia of the idiopathic type or that due to lupus erythematosus 10. 12 Acthar Gel may be used for the. FSGS RETROSPECTIVE STUDY Madan et al 2016 FSGS Retrospective Study Alhamad et al 2019 FSGS PROSPECTIVE STUDY Tumlin et.
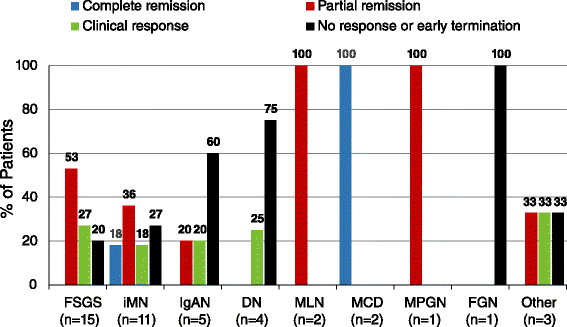
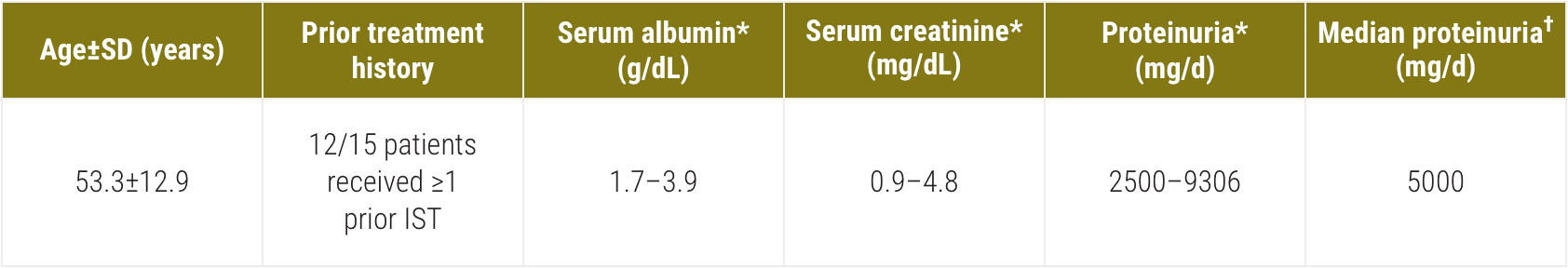
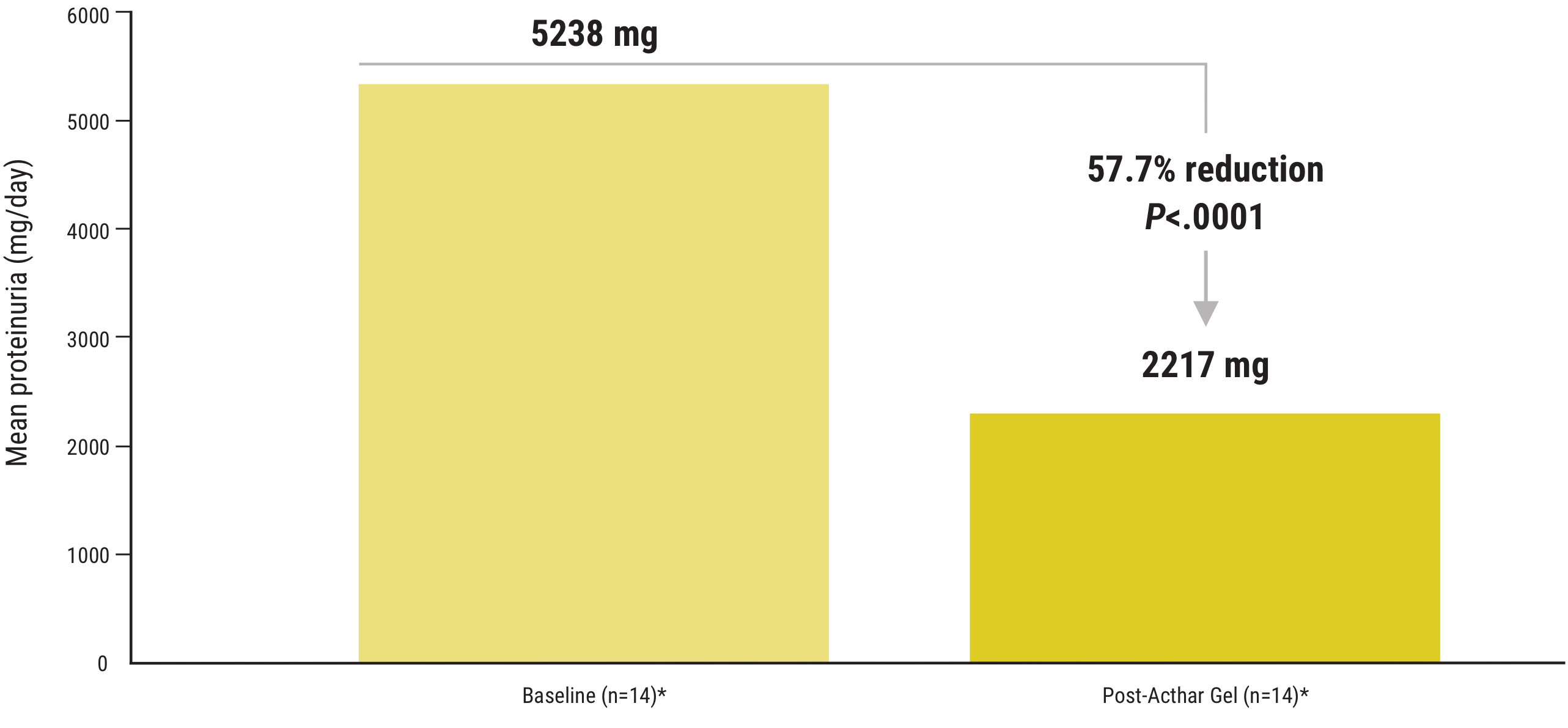
Acthar Gel commonly known as Acthar is a prescription medication that is FDA approved for the treatment of proteinuria associated with Nephrotic Syndrome. At room temperature it changes to liquid form ready for injection. NS etiologies included idiopathic focal segmental glomerulosclerosis FSGS 15 idiopathic membranous.
Acthar Gel a form of ACTH therapy is a highly purified form of ACTH and is delivered as a gel to provide extended release of ACTH following injection. Acthar Gel Study - Nephrotic syndrome and FSGS. It was initially FDA-approved in 1952 for the reduction of proteinuria and hyperlipidemia associated with childhood nephrotic syndrome NS.
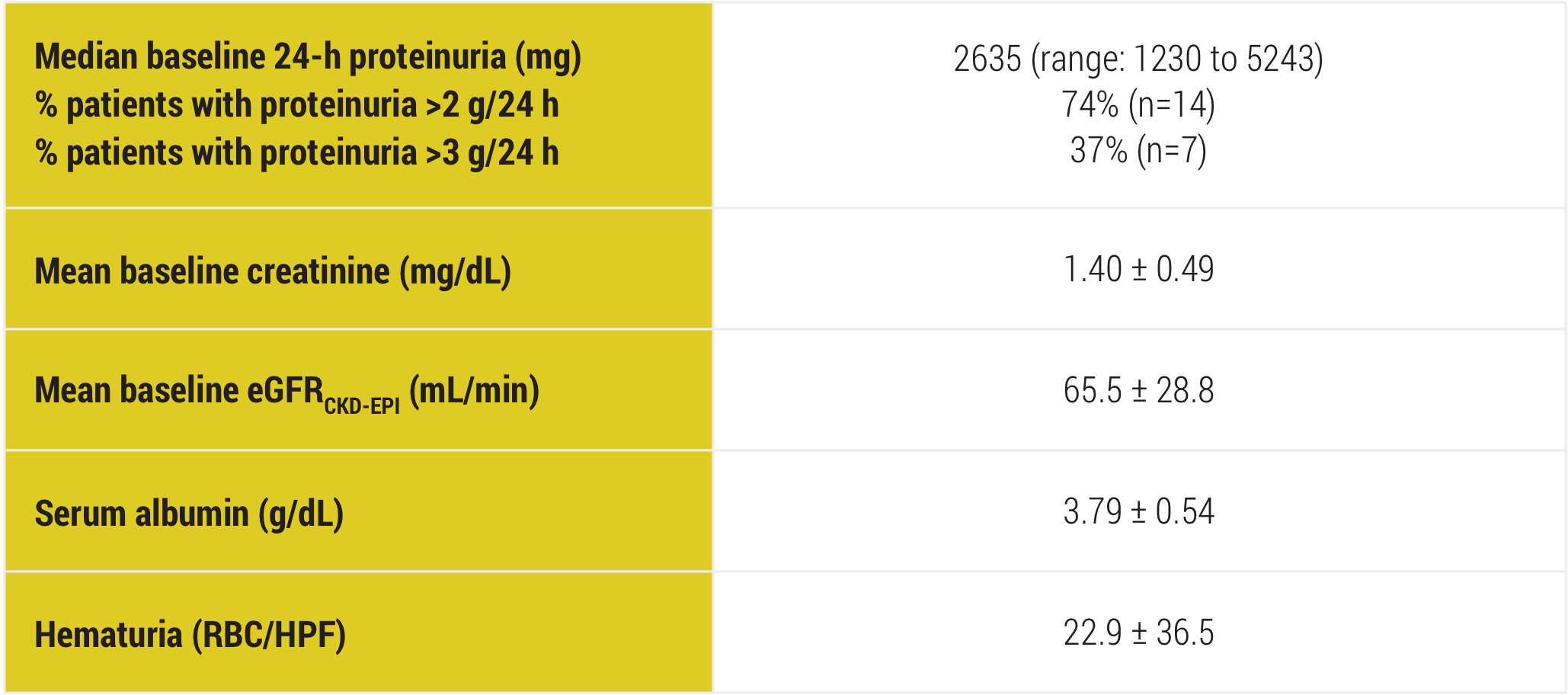
Zand L Canetta P Lafayette R Aslam N Jan N Sethi S Fervenza FC Kidney International Reports 2020. Why havent I heard of. She had manifestations of severe nephrotic syndrome with a serum albumin level of 25 mgdL and anasarca.
FSGS is a condition where you have scarring in the special areas of the kidney that filter the blood. Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age.
She started treatment with Acthar Mallinckrodt Pharmaceuticals repository corticotropin injection gel 80 mg twice weekly.
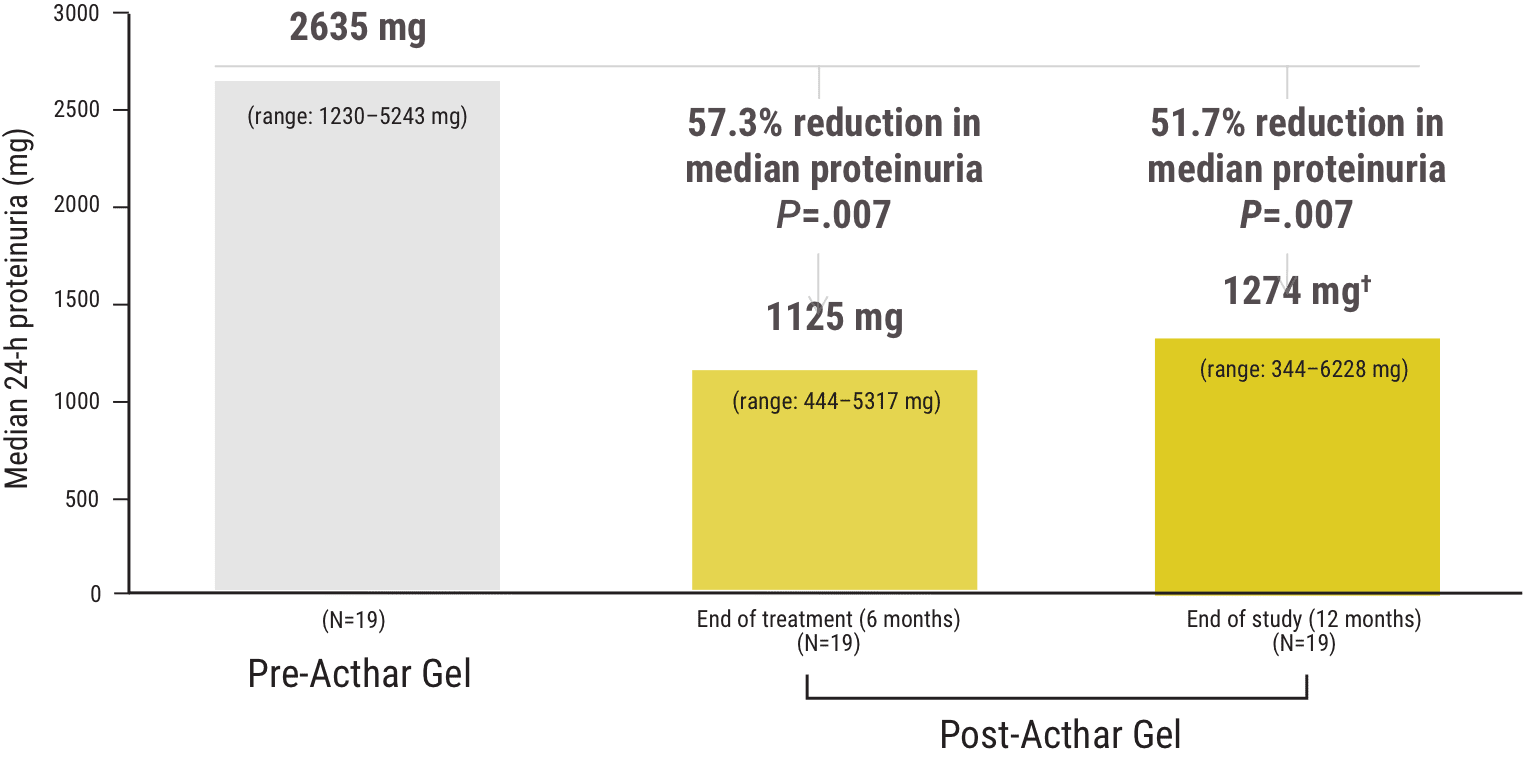
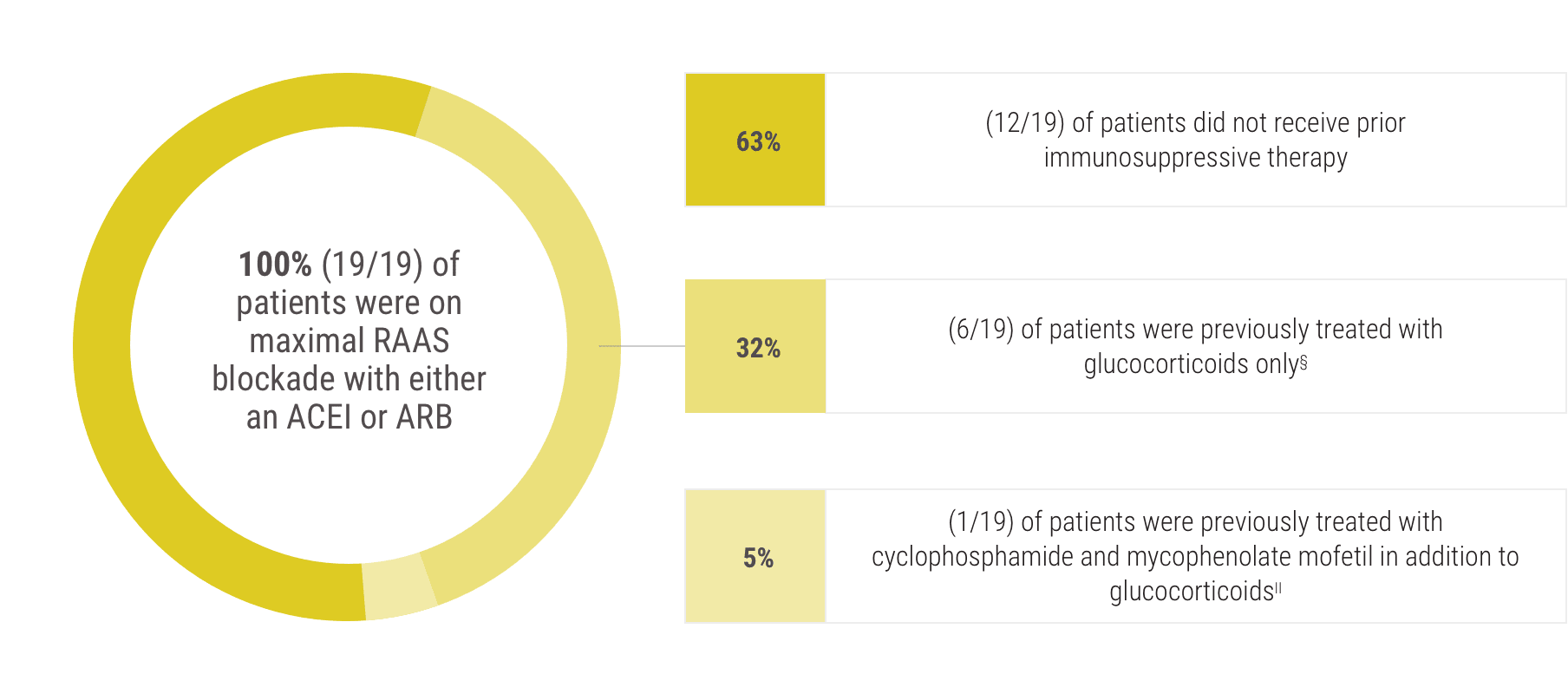
Acthar Gel commonly known as Acthar is a prescription medication that is FDA approved for the treatment of proteinuria associated with Nephrotic Syndrome. Lowing Acthar gel treatment has been shown in patients with idiopathic membranous nephropathy iMN idio-pathic focal segmental glomerulosclerosis FSGS IgA nephropathy IgAN minimal change disease MCD and diabetic nephropathy DN 1420. FSGS RETROSPECTIVE STUDY Madan et al 2016 FSGS Retrospective Study Alhamad et al 2019 FSGS PROSPECTIVE STUDY Tumlin et. She had manifestations of severe nephrotic syndrome with a serum albumin level of 25 mgdL and anasarca. Inducing a diuresis or a remission of proteinuria in nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosus Monotherapy for the treatment of infantile spasms in infants and children under 2 years of age Treatment of acute exacerbations of multiple sclerosis in adults. 12 Acthar Gel may be used for the. Acthar Gel is indicated as monotherapy for the treatment of infantile spasms in infants and children under 2 years of age. IMMUNOGLOBULIN A NEPHROPATHY I g AN Acthar Gel may help appropriate patients with proteinuria in nephrotic syndrome due to IgAN Image is not an actual patient. Reduction of proteinuria in people with nephrotic syndrome of the idiopathic type unknown origin without uremia accumulation of urea in the blood due to malfunctioning kidneys or that due to lupus erythematosus lupus Treatment of infantile spasms in infants and children under 2 years of age.
She started treatment with Acthar Mallinckrodt Pharmaceuticals repository corticotropin injection gel 80 mg twice weekly. Why havent I heard of. Acthar Gel may help appropriate patients with proteinuria in nephrotic syndrome due to FSGS Image is not an actual patient. This multicenter retrospective case series included adult patients with NS N 44 treated with Acthar gel at 6 clinical practices. She had manifestations of severe nephrotic syndrome with a serum albumin level of 25 mgdL and anasarca. Acthar Gel is currently the only Food and Drug Administration therapy approved for the treatment of nephrotic syndrome. Conclusions Acthar gel may meet an important treatment need in patients with treatment-resistant NS in response to first-line therapies patients unable to tolerate first-line therapies and in.


































Posting Komentar untuk "Acthar Gel Nephrotic Syndrome"